Partnering for Performance with Prompt Results
NMS Labs provides bioanalytical and specialty laboratory services to clinical researchers, sponsors, and clinical research organizations throughout all aspects of the product development cycle. With over 2,500 assays and our ability to develop customizable methods, NMS Labs can help you achieve your clinical studies on time and within budget.
How NMS Labs Addresses Sponsors’ Needs:
- Data Quality is a priority
- On-time delivery of test results
- Scientific expertise in multiple therapeutic areas
- Novel assay validations
- Access to real-time data
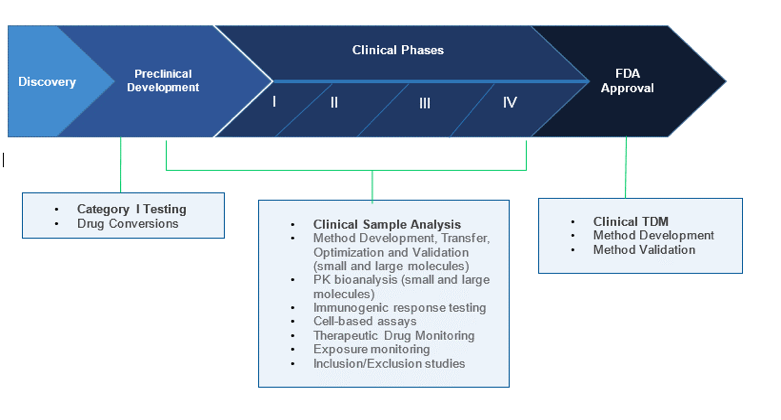
Bioanalytical Support Services
- Method development, transfer, optimization and validation
- Pharmacokinetic (PK) bioanalysis
- Immunogenic response testing
- Cell-based assays
- Therapeutic Drug Monitoring
- Exposure monitoring
- Biomarker development and commercialization
- Inclusion/Exclusion studies
- Customized testing and reporting
Case Study: Method Development and Validation of a Novel Antipsychotic for a Clinical Study
Executive Summary
A large pharmaceutical company required a bioanalytical therapeutic drug monitoring assay for a novel antipsychotic. The sponsor required the assay to be developed, validated, and submitted to the appropriate regulatory bodies within four months prior to the initiation of a Phase II study. NMS Labs completed this within the four months, allowing the sponsor to initiate the clinical study without delay.
Challenges
The sponsor contracted with a large clinical research organization with their own central laboratory to provide bioanalytical support services for the clinical study. The CRO’s central laboratory did not have the capability of developing the test within the sponsor’s timeframe and contracted with NMS Labs to perform the service.
Outcomes
- NMS Labs helped the sponsor initiate the clinical study without delay
- The sponsor had real time access to the clinical data which helped them to monitor the progress of the study and implement changes
To learn why 7 out of 10 CROs use NMS Labs as their partner of choice for critical studies, contact us at 1-866-522-2206 / CSTIQ@nmslabs.com